A centralized digital log captures every preventive and corrective maintenance action—timestamped and attributed—replacing scattered spreadsheets and paper forms.
Targeted QC support to fill gaps left by your LIS — including centralized Levey-Jennings chart storage to streamline audits and inspections.
Platform dashboard that centralizes all lab assignments—complete with deadlines, status updates, and ownership—ensuring full visibility and accountability without tasks slipping through gaps



Accountability and visibility enables teams to document, track, and resolve issues in real-time while providing leadership with transparent oversight into performance, compliance, and operational metrics. LabLogs empowers laboratory teams and leadership with secure, remote access to operational data, compliance records, and performance dashboards from any location.







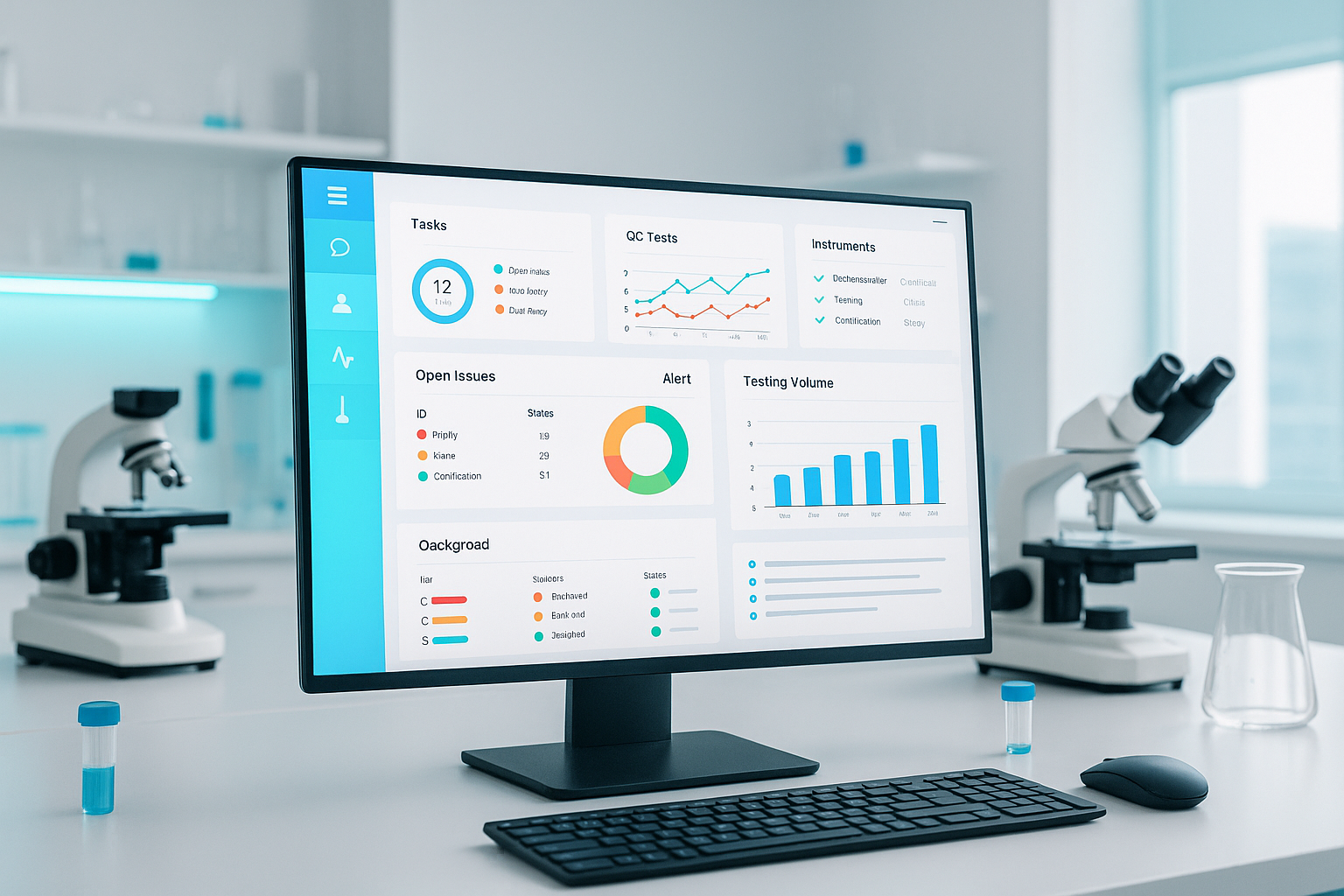
Schedule and manage maintenance tasks to reduce equipment downtime and ensure compliance.
Standardized QC documentation for all test systems, ensuring consistency and accuracy.
Track the lifecycle of lots and reagents from receipt to expiration with complete traceability.
Minimize downtime with detailed repair history and operational status reports.
Monitor and maintain lab conditions to guarantee accurate results.
Automate routine tasks and focus on what matters.
Stay compliant and avoid costly citations.
Streamlined workflows improve testing capacity.

(888) 4000-2424

LabLogs was founded to address a widespread challenge in laboratory operations: the inefficiencies, inconsistencies, and compliance risks associated with manual tracking of maintenance and quality control tasks. Jeremy recognized the need for a more reliable and standardized approach and partnered with Daniel Summers to develop a solution that brings clarity, consistency, and accountability to lab documentation.
What began as an internal solution in Jeremy’s own laboratories has since evolved into a robust, scalable platform now trusted by labs across the country. By enabling real-time tracking, automated alerts, and centralized records, LabLogs empowers lab teams to improve compliance, reduce errors, streamline workflows, and ensure readiness for inspections—all while enhancing visibility for leadership and simplifying daily operations.

(844) LAB-LOGS